
Dermal Filler Complications: What Should You Know?
By Kaitlyn Konsur , RN In recent years, the popularity of dermal fillers has increased, promising satisfying results for fine lines, wrinkles, and facial volume loss. With minimal downtime
Tuesday, August 02, 2011
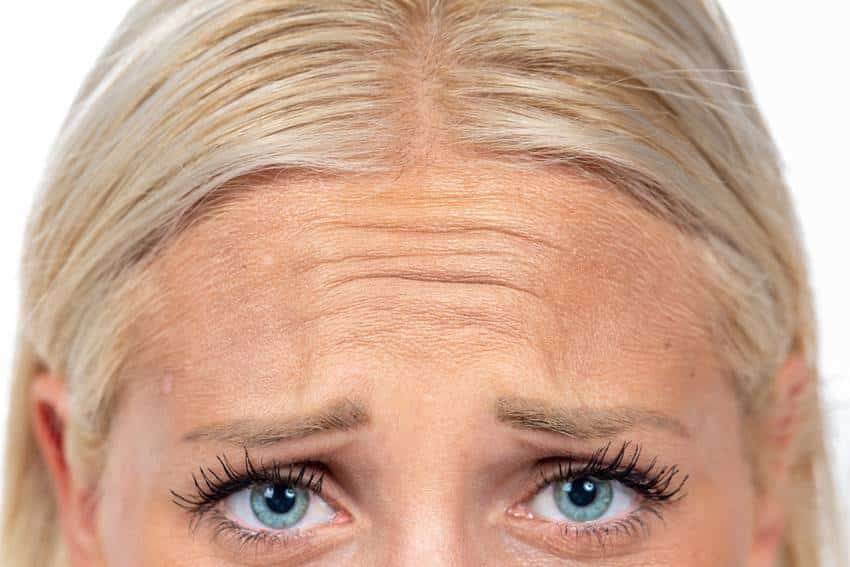
Washington — The Food and Drug Administration (FDA) has approved Xeomin® (incobotulinumtoxinA, Merz Aesthetics) for temporary improvement of moderate to severe glabellar lines, Medscape Today reports.
According to a Merz statement, the approval is based on the results of two multicenter U.S. clinical trials involving 547 healthy adults. In both studies, Xeomin® injections significantly improved the appearance of glabellar lines in 30 days when compared with placebo. Headache was the most common adverse reaction observed.
Under the brand name Bocouture, Xeomin® is already approved in 14 European countries including Germany, the United Kingdom, France, Italy and Spain. It was FDA-approved in 2010 for the treatment of cervical dystonia and blepharospasm.
https://www.facialesthetics.org/blog/fda_oks_xeomin_for_frown_lines

By Kaitlyn Konsur , RN In recent years, the popularity of dermal fillers has increased, promising satisfying results for fine lines, wrinkles, and facial volume loss. With minimal downtime

By Sydney Gatta, RN In the realm of facial aesthetics and rejuvenation, innovative treatments continue to emerge, offering patients a plethora of options to enhance their appearance and combat

By Arianna Bankovich, RN Introduction: Botox, derived from the bacterium Clostridium botulinum, has long been renowned for its cosmetic applications in reducing wrinkles and fine lines. However, a growing body